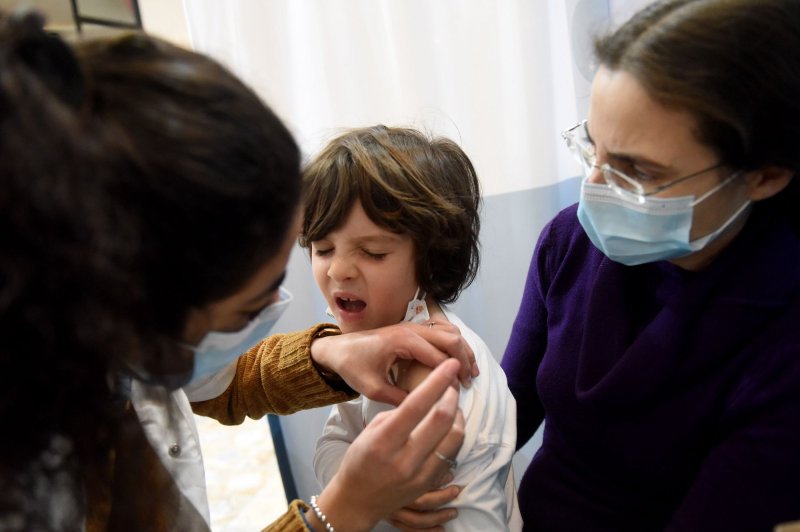
1 of 4 | The FDA said Thursday it has approved emergency use of Moderna and Pfizer-BioNTech bivalent boosters for kids 6 months and older. File Photo by Debbie Hill/UPI |
License Photo
Dec. 8 (UPI) -- The U.S. Food and Drug Administration Thursday amended emergency use authorization for Pfizer and Moderna bivalent COVID-19 vaccines to include kids 6 months and older.
"More children now have the opportunity to update their protection against COVID-19 with a bivalent COVID-19 vaccine, and we encourage parents and caregivers of those eligible to consider doing so -- especially as we head into the holidays and winter months where more time will be spent indoors," said FDA Commissioner Robert M. Califf, M.D., in a statement.
Califf said the more people keep current with COVID-19 vaccines, the more benefit there will be for families, individuals and public health by preventing severe illnesses, hospitalizations and deaths.
Pfizer asked the FDA to make this authorization earlier this month.
The FDA said kids ages 6 months through 5 years who have received the original Moderna vaccine can now get a single updated bivalent Moderna booster two months after getting the primary vaccine series.
Kids 6 months to 4 years old who have not begun their three-dose primary series Pfizer-BioNTech COVID-19 vaccine or haven't gotten a third dose can now get the Pfizer-BioNTech bivalent vaccine booster as a third dose in their primary series.
Children 6 months through 4 years old who have completed their three-dose Pfizer-BioNTech vaccine series will not be eligible for the Pfizer-BioNTech bivalent vaccine. The FDA expects to receive data on the updated booster for these children in January and will review it as quickly as possible.
According to the FDA, both the Moderna and the Pfizer-BioNTech bivalent vaccines include an mRNA component corresponding to the original COVID-19 strain and an mRNA component corresponding to the Omicron variants BA.4 and BA.5 lineages.
In November CDC data showed that those lineages have been replaced by the BQ.1.1 and BQ.1 COVID-19 variants as the dominant strains circulating in the United States.
But Johns Hopkins University virologist Andrew Pekosz said since virtually all U.S. COVID-19 varinats are related to BA.5, the bivalent vaccines should still increase immunity to some extent.
Peter Marks, M.D., Ph.D., director of the FDA's Center for Biologics Evaluation and Research said in a statement: "Vaccines remain the best defense against the most devastating consequences of disease caused by the currently circulating Omicron variant, such as hospitalization and death. Based on available data, the updated, bivalent vaccines are expected to provide increased protection against COVID-19."