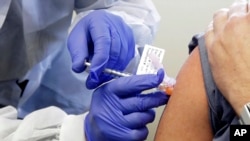
U.S. health officials say the first human trial has begun to test an experimental coronavirus vaccine, as scientists race to find treatments for the pandemic.
Scientists at the Kaiser Permanente Washington Research Institute in Seattle gave the first shots Monday to a small group of healthy people.
The U.S. National Institutes of Health (NIH) said the trial would involve 45 healthy adult volunteers ages 18 to 55 years old who would be given the experimental vaccine over a six-week period.
One of the test subjects, Jennifer Haller, 43, of Seattle, told the Associated Press, "This is an amazing opportunity for me to do something.'' She said her two teenage children “think it’s cool” that she is taking part in the trial.
The vaccine was developed by scientists at NIH and the biotechnology company Moderna, which is based in Cambridge, Massachusetts.
It is one of many studies of experimental vaccines that will take place around the world in the coming months to try to find a way to protect people against COVID-19.
Scientists say that a vaccine will likely not be available for widespread use for another 12-18 months, as all potential vaccines must go through several phases of testing to prove they works and are safe.
Despite the time investment, Anthony Fauci, head of infectious diseases at the NIH, said in a statement Monday, "Finding a safe and effective vaccine to prevent infection with SARS-CoV-2 is an urgent public health priority."
"This Phase 1 study, launched in record speed, is an important first step toward achieving that goal," he added.
Researchers say there is no risk of the patients in the trial contracting coronavirus because the injections do not contain the actual virus.
They say the experimental vaccine contains only part of the virus’ genetic code pertaining to a protein called the “spike'' which protrudes from the surface of the new coronavirus and allows the virus to invade human cells.
Scientists are focusing their research on that protein, because if they can block it, then people will not get infected.
The experimental vaccine will help the body produce harmless spike proteins, which will hopefully prime the subject’s immune system to attack such proteins, and thereby be ready to fight coronavirus.
Scientists say they will carefully monitor the test subjects to see if the experimental vaccine is inducing an immune response and will check for any potential side effects.